
There has been a continuous race between discoveries of new diseases and their best treatments. On the one hand, discovery of new diseases is a challenging for scientists, while on the other, discovering their treatment is very tough job for them. Because it takes a lot of time and experiments to discover new treatments. Medical scientists are striving their best to discover new ways of treatment of existing diseases. Not all the diseases have their complete treatment discovered; yet some diseases (like high blood pressure & diabetes) are still on management category. It’s a great achievement if patient, suffering from a life threatening disease, recovered normal life by using simple medication. FDA approves new evidence that Cosentyx® inhibits Scalp Psoriasis. Cosentyx ® contains Secukinumab as active ingredient which is first interleukin-17A (IL-17A) inhibitor.
FDA approved the label update of Cosentyx® in moderate-to-severe plaque psoriasis. The updated label includes Cosentyx® data in moderate-to-severe scalp psoriasis – one of the difficult-to-treat forms of the disease, which affects approximately half of all psoriasis patients.[1][2]
FDA Approvals for Secukinumab:
- In January 2015, FDA approved secukinumab to treat adults with moderate-to-severe plaque psoriasis.
- In January 2016, the FDA approved it to treat adults with ankylosing spondylitis, and psoriatic arthritis.
- While in February 2018, FDA approved a label update to include that Secukinumab inhibits moderate-to-severe scalp psoriasis.
What is Plaque Psoriasis?
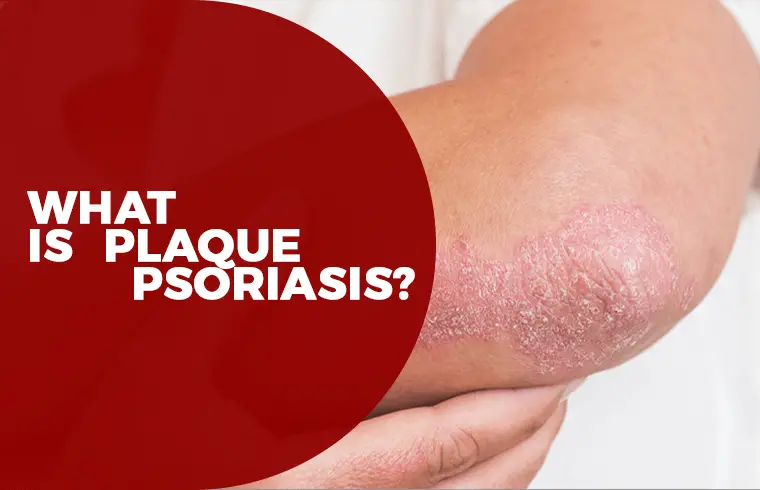
Plaque Psoriasis is most common (about 80%-90%) form of Psoriasis in which raised, red patches covered with a silvery white buildup of dead skin cells or scale. On skin, the discoloration is darker and thicker, more of a purple or grayish color or darker brown.
These patches or plaques most often appear on the scalp, knees, elbows and lower back. They range from coin-size to palm-size. They are often itchy and painful, and they can crack and bleed.3
What is Psoriatic Arthritis?
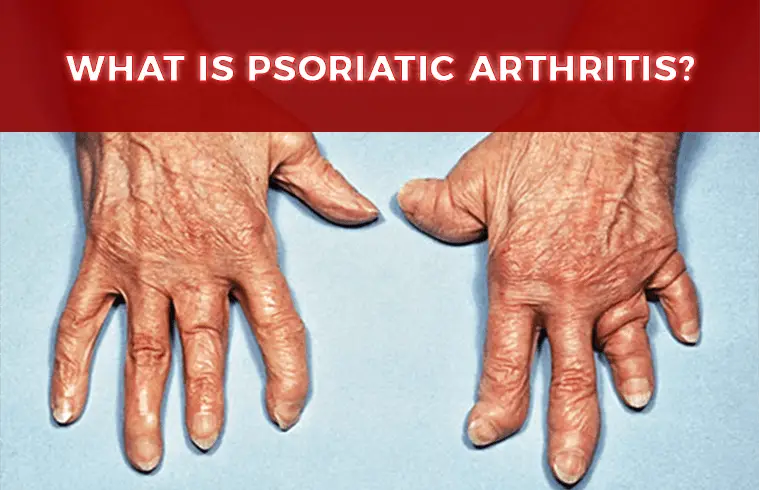
Psoriatic patients having the symptoms of arthritis- that is joint’s pain and inflammation. Up to 30 percent of people with psoriasis can also develop psoriatic arthritis.
What is Ankylosing Spondylitis?

Ankylosing Spondylitis is a form of arthritis that primarily affects the spine, although other joints can become involved. It causes inflammation of the spinal joints (vertebrae) that can lead to severe, chronic pain and discomfort.4
What is Scalp psoriasis?
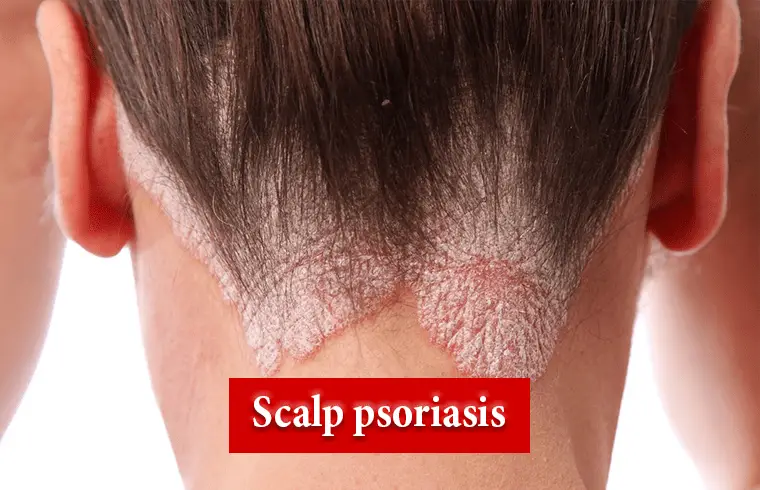
Scalp psoriasis is a common skin disorder that makes raised, reddish, often scaly patches. It can pop up as a single patch or several, and can even affect your entire scalp. It can also spread to your forehead, the back of your neck, or behind and inside your ears.5
What is their Management?
Cosentyx® (Secukinumab)
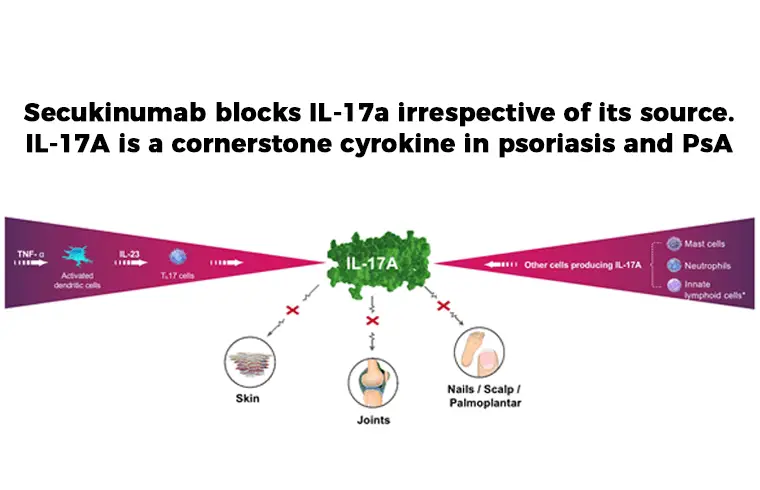
One of the treatment choice is: Secukinumab is a human IgG1κ monoclonal antibody that binds to, and inhibit, protein interleukin (IL)-17A (pro-inflammatory cytokine). It is used to inhibit Plaque Psoriasis, Ankylosing Spondylitis and Psoriatic Arthritis.
What are Monoclonal Antibodies?
Monoclonal antibodies (mAb or moAb) are antibodies that are made by identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies have affinity, in that they bind to the same epitope (the part of an antigen that is recognized by the antibody).6
How it works?
Secukinumab is a human IgG1 monoclonal antibody that selectively binds to the interleukin-17A (IL-17A) cytokine and inhibits its interaction with the IL-17 receptor. IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. Secukinumab inhibits the release of proinflammatory cytokines and chemokines.
Dosage and Administration
It is given by subcutaneous injection and is sold in a pre-filled syringe or auto-injector that can be used at home and as a lyophilized powder for use in hospitals and clinics.
In some counties, it’s available in vial with powder for solution which is reconstituted before administration.
1- Plaque Psoriasis
Recommended dosage is 300 mg by subcutaneous injection at weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks. For some patients, a dose of 150 mg may be acceptable.7
2- Psoriatic Arthritis
• For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, use the dosage and administration for plaque psoriasis.
• For other psoriatic arthritis patients administer the recommended dosage:
With a loading dosage- is 150 mg at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
Without loading dosage- is 150 mg every 4 weeks.
If a patient continues to have active psoriatic arthritis, consider a dosage of 300 mg.7
3- Ankylosing Spondylitis
• Administer with or without a loading dosage as for Psoriatic Arthritis.
Caution:
- This drug inhibits part of immune system, so do not use if you are immuno-compromised.
- Trials are not conducted on Pregnant women, so before going to take this medicine, avoid pregnancy.
FAQs:
Can I use the Cosentyx ?
Use this medication with the supervision of qualified person and only on qualified doctor's advice.
For How long it would be taken?
Because it is for auto-immune diseases and these can only be managed, so Cosentyx can be taken for life long to manage the disease.
Can hepatitis patient take Cosentyx?
Caution should be taken in Hepatitis patients. Better to consult with Dermatologist before going to take Cosentyx.
Can renal impaired patient take Cosentyx?
There is no study available on renal impaired patients.
References:
[1]- Cosentyx [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. [2]- National Psoriasis Foundation. Scalp Psoriasis. psoriasis.org. https://www.psoriasis.org/about-psoriasis/specific-locations/scalp [3]- Psoriasis.org [4]- Spondylitis.org [5]- webmd.com [6]- wikipedia.org [7]- fda.gov7 Steps to Prepare for a doctor’s Appointment

Can You Sue a Doctor for Wrong Diagnosis






This blog FDA APPROVES NEW EVIDENCE THAT COSENTYX® INHIBITS SCALP PSORIASIS helped me
with my grandmother’s Psoriasis. Hope you heal quickly!
Kiss you all!
This FDA APPROVES NEW EVIDENCE THAT COSENTYX® INHIBITS SCALP PSORIASIS site has helped me
many times in health problems. Kiss you all!
It wrote his thoughts while reading the article amazingly
🙂
Best regards,
Balle Valenzuela